Need More Hydrochloric Acid? Your Comprehensive Guide
Hydrochloric acid (HCl) is a potent chemical with a wide array of applications, from industrial processes to laboratory experiments. Its powerful properties make it indispensable for various tasks, but also necessitate careful handling and acquisition. This comprehensive guide dives deep into the methods of obtaining more hydrochloric acid, addressing crucial safety concerns, and providing practical advice for both professionals and hobbyists.
Understanding how to procure additional hydrochloric acid requires a careful balance of resourcefulness and responsible practice. Whether you're a seasoned chemist or simply exploring the world of chemistry, a thorough understanding of the acquisition process is essential.
The journey to obtain more HCl begins with recognizing its corrosive nature and the importance of safety precautions. From selecting the appropriate supplier to understanding storage requirements, every step demands careful consideration. This guide serves as a roadmap, navigating the intricacies of obtaining this powerful chemical.
Historically, hydrochloric acid has been a cornerstone in various industries, playing a vital role in processes ranging from metal cleaning to pH regulation. Its importance stems from its remarkable ability to react with various substances, making it a crucial component in numerous chemical reactions.
However, acquiring more hydrochloric acid isn't simply about knowing where to find it. It also necessitates a deep understanding of responsible handling, proper storage, and potential hazards. This guide addresses these crucial aspects, ensuring you're equipped with the knowledge to utilize HCl safely and effectively.
Hydrochloric acid can be obtained through various channels. Chemical supply companies are a primary source, catering to both industrial and individual needs. Additionally, some hardware stores carry diluted HCl for household applications like cleaning. However, concentrations vary, and understanding your specific requirements is crucial.
When dealing with hydrochloric acid, safety should always be the top priority. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat or apron. Work in a well-ventilated area to minimize exposure to fumes.
Three key benefits of having access to hydrochloric acid include its versatility in various applications, its effectiveness as a cleaning agent, and its role in pH adjustment. For instance, in industrial settings, HCl is used in metal processing, while in the home, it can be used to clean stubborn stains. It's also essential for maintaining the correct pH balance in swimming pools and other water systems.
Creating an action plan for obtaining more hydrochloric acid requires careful consideration. First, identify your specific needs and the required concentration. Next, research reputable suppliers and compare their offerings. Finally, ensure you have the necessary safety measures in place before the acid arrives.
Advantages and Disadvantages of Obtaining More Hydrochloric Acid
| Advantages | Disadvantages |
|---|---|
| Essential for various industrial and domestic applications. | Highly corrosive and requires careful handling. |
| Effective cleaning agent. | Potential health hazards if mishandled. |
| Crucial for pH adjustment. | Storage requires specific conditions. |
Best practices for handling hydrochloric acid include always wearing PPE, working in a well-ventilated area, storing the acid in a designated area away from incompatible materials, and having a spill kit readily available.
Challenges in obtaining hydrochloric acid might include sourcing a reliable supplier, navigating regulations for purchasing concentrated solutions, and ensuring safe transportation. Solutions involve thorough research, obtaining necessary permits, and partnering with reputable transportation companies.
Frequently Asked Questions:
1. Where can I buy hydrochloric acid? Answer: Chemical supply companies and some hardware stores.
2. What safety precautions are necessary? Answer: Always wear PPE, work in a well-ventilated area, and store appropriately.
3. What are the different concentrations available? Answer: Concentrations vary depending on the application and supplier.
4. What is the shelf life of hydrochloric acid? Answer: The shelf life varies depending on storage conditions.
5. How should I dispose of hydrochloric acid? Answer: Follow local regulations for proper disposal.
6. What are the first aid procedures for HCl exposure? Answer: Immediately flush affected area with water and seek medical attention.
7. Can I dilute concentrated hydrochloric acid? Answer: Yes, but always add acid to water, never the reverse.
8. Is it legal to purchase hydrochloric acid? Answer: Regulations vary; check local laws and regulations.
Tips and tricks for working with hydrochloric acid include using a fume hood whenever possible, having a neutralizing agent readily available, and labeling containers clearly.
In conclusion, understanding how to obtain more hydrochloric acid is a process that necessitates meticulous planning and unwavering adherence to safety protocols. From sourcing the chemical to implementing best practices for its usage, each step plays a crucial role in ensuring both efficacy and safety. The benefits of having access to this potent chemical are numerous, from its industrial applications to its usefulness in household cleaning. However, the potential hazards associated with improper handling underscore the importance of rigorous safety measures. By following the guidelines outlined in this guide, individuals can confidently and safely utilize hydrochloric acid for their specific needs. Remember, prioritizing safety is paramount when working with this powerful substance. Always consult with safety data sheets and relevant regulations before handling hydrochloric acid. By taking proactive steps and emphasizing safety, you can harness the power of hydrochloric acid while mitigating potential risks.

Smith Wigglesworth Quote I can get more out of God by believing Him | Kennecott Land

The Fed Cannot Fix Todays Energy Inflation ProblemOur Finite World | Kennecott Land

Hydrochloric Acid Packaging Type Bottle Grade Standard Industrial | Kennecott Land

Unique Ammonia And Hydrogen Chloride Gases Are Mixed Chemical Equation | Kennecott Land

Hydrochloric Acid 32 Grade Standard Industrial Grade at Rs 500ton | Kennecott Land

how to get more hydrochloric acid | Kennecott Land

Hydrochloric Acid Hcl For Industrial Pure Rs 12 kg United Chemical | Kennecott Land

how to get more hydrochloric acid | Kennecott Land

Here are 5 easy ways to help fix your gut health and fat digestion | Kennecott Land
:max_bytes(150000):strip_icc()/ph-of-the-stomach-608195_final-da87214880ac4716bd5e570b31601029.png)
Alguna vez se preguntó cuál es el pH dentro de su estómago aquí está | Kennecott Land

science chemistry hydrochloric acid label | Kennecott Land

how to get more hydrochloric acid | Kennecott Land

Hcl acid or base | Kennecott Land
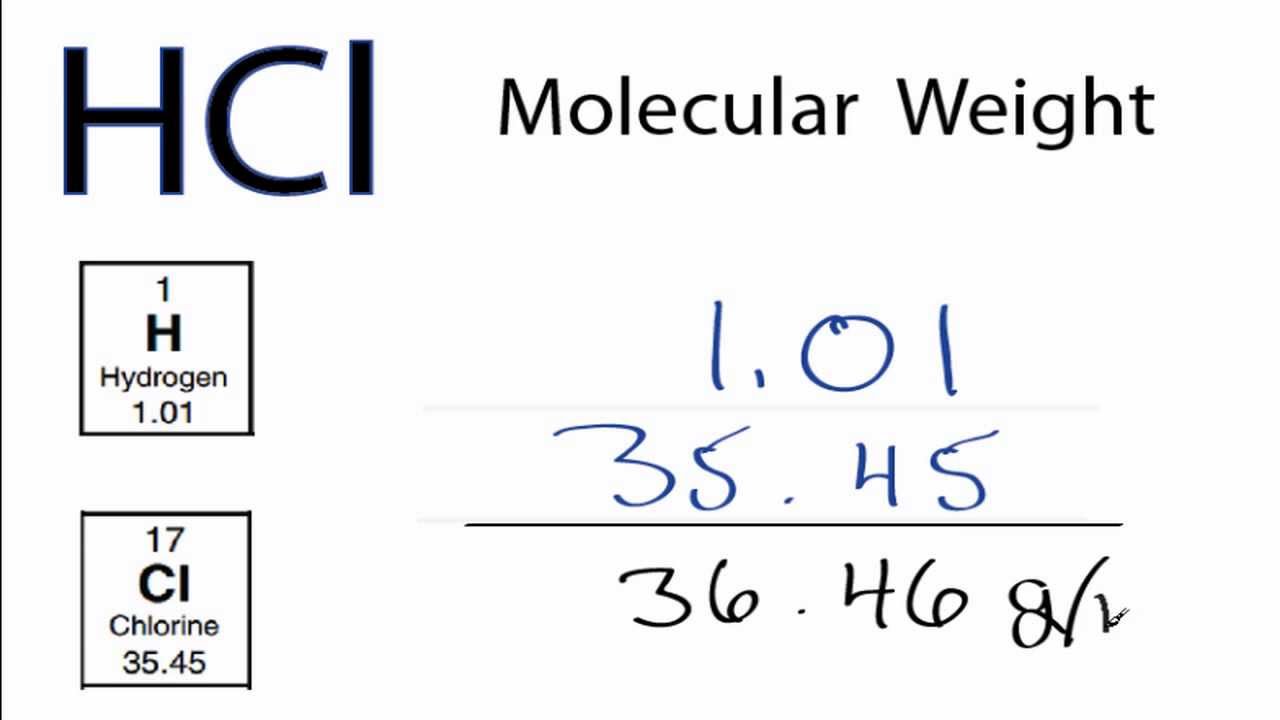
Útil Descifrar Aparador peso molecular hcl Usando una computadora | Kennecott Land

Hydrochloric Acid Warning Signs | Kennecott Land